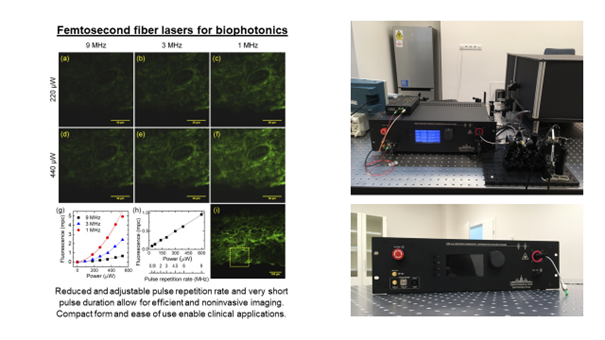
Femtosecond lasers are used in many techniques in biophotonics, including multiphoton microscopy, two-photon fluorescence ophthalmoscopy, and two-photon microperimetry. These methods require precisely selected parameters of ultrashort pulses to ensure noninvasive and efficient imaging of the sample under study or examination of the patient. Titanium-sapphire lasers or parametric oscillators are most commonly used for this purpose. However, these laser sources are very expensive, complicated to use, require water cooling, and are not mobile. A solution to these problems may be using femtosecond fiber lasers, which offer equally short pulse durations but are much more compact and easier to use and transport, enabling clinical translation. The lasers are being developed at the Wrocław University of Science and Technology, and researchers at ICTER are working on their applications.
The first application is multiphoton microscopy, particularly fluorescence microscopy with two-photon excitation. In this application, it is crucial to minimize the average excitation power of the laser, thus reducing the thermal interaction with the sample under study. For this purpose, a femtosecond fiber laser with an adjustable repetition rate and very short pulse duration (less than 60 fs) was developed. The laser operates in the near-infrared spectral range (central wavelength around 780 nm) by doubling the frequency of a laser doped with erbium ions (operating at 1560 nm). The laser was developed as a compact, easy-to-use prototype [1].
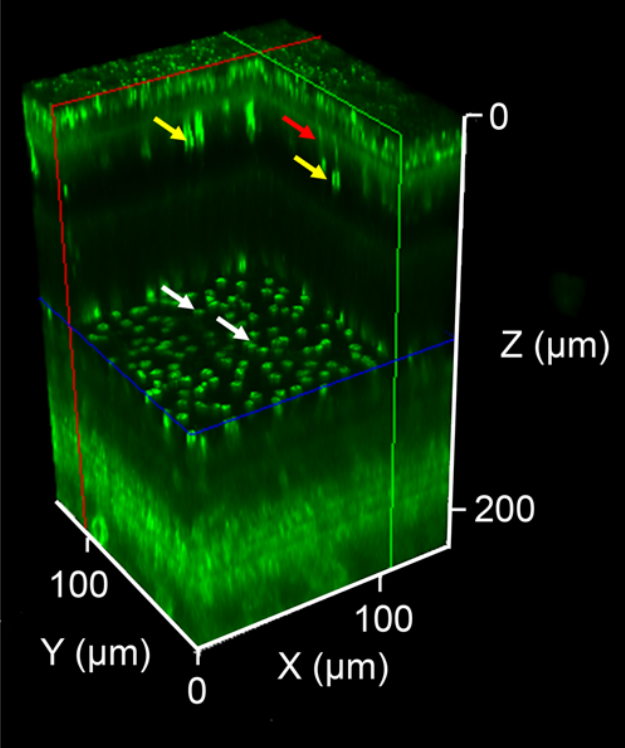
The second application, from the field of ophthalmology, is two-photon fluorescence ophthalmoscopy. This method allows noninvasive imaging of autofluorescence induced in the retina and retinal pigment epithelial layer. In this application, the key is to reduce the power of the average laser beam used to excite the fluorescence. This has been achieved by using a femtosecond fiber laser with an adjustable repetition rate and a very short pulse duration [2].
A recent application, also from the field of ophthalmology, is two-photon microperimetry and the study of the phenomenon of two-photon vision. Another femtosecond fiber laser with a tunable wavelength, ranging from 872 to 1075 nm, was developed for this purpose. Such a wide tuning range allowed a better study of humans’ scotopic spectral sensitivity of two-photon vision [3].
Autor: Jakub Bogusławski, PhD
Team:
Jakub Bogusławski, PhD jboguslawski@ichf.edu.pl
Marcin Marzejon, PhD mmarzejon@ichf.edu.pl
Katarzyna Komar, PhD kkomar@ichf.edu.pl
Prof. Maciej Wojtkowski mwojtkowski@ichf.edu.pl
Publications:
- D. Stachowiak, J. Bogusławski, A. Głuszek, Z. Łaszczych, M. Wojtkowski, G. Soboń, “Frequency-doubled femtosecond Er-doped fiber laser for two-photon excited fluorescence imaging,” Biomedical Optics Express 11(8), 4431 (2020).
- Jakub Boguslawski, Grazyna Palczewska, Slawomir Tomczewski, Jadwiga Milkiewicz, Piotr Kasprzycki, Dorota Stachowiak, Katarzyna Komar, Marcin J Marzejon, Bartosz L Sikorski, Arkadiusz Hudzikowski, Aleksander Głuszek, Zbigniew Łaszczych, Karol Karnowski, Grzegorz Soboń, Krzysztof Palczewski, Maciej Wojtkowski, “In vivo imaging of the human eye using a two-photon excited fluorescence scanning laser ophthalmoscope,” The Journal of Clinical Investigation 2022;132(2):e154218.
- Dorota Stachowiak, Marcin Marzejon, Jakub Bogusławski, Zbigniew Łaszczych, Katarzyna Komar, Maciej Wojtkowski, Grzegorz Soboń, “Femtosecond Er-doped fiber laser source tunable from 872 to 1075 nm for two-photon vision studies in humans,” Biomedical Optics Express 131(4), 1899-1911 (2022).