Diffuse optics offers a noninvasive portable approach for examining biological tissues, including the human brain, in vivo [1]. Near-infrared spectroscopy (NIRS) [2] and diffuse correlation spectroscopy (DCS) are the primary diffuse optical modalities [3, 4]. In both approaches, the light illuminates the tissue, and diffusively scattered photons are collected at some distance from the emitter (typically 2-3 cm). NIRS uses the detected signal to estimate optical properties (absorption and scattering), while DCS quantifies the blood flow from temporal changes of the remitted light intensity. Although these methods have been applied to monitor brain oxygenation and blood flow, their most widely adopted versions rely on continuous wavelength (CW) lasers, precluding absolute measures of the optical and dynamical tissue properties [5].
Time-domain NIRS (TD-NIRS) enables quantification of the optical properties through the sample’s photon time-of-flight distribution (TOF distribution) [9-12]. This capability can also be combined with correlation spectroscopy to achieve TOF- (or photon path-length-) resolved blood flow information. So, we could better distinguish photons traversing superficial layers (short TOFs) from photons traveling deep into the brain (long TOFs). Also, given the TOF distribution, we could estimate the optical properties to achieve the absolute blood flow index (BFI), effectively combining the capabilities of TD-NIRS with DCS into a single modality. Such an approach is called time-domain diffuse correlation spectroscopy (TD-DCS) [6, 7].
Though TD-DCS is a powerful technique even applied in clinics, it relies only on light intensity. Therefore, TD-DCS does not enable the detection of the optical phase [8]. The optical phase is accessible in interferometric near-infrared spectroscopy (iNIRS) [9].
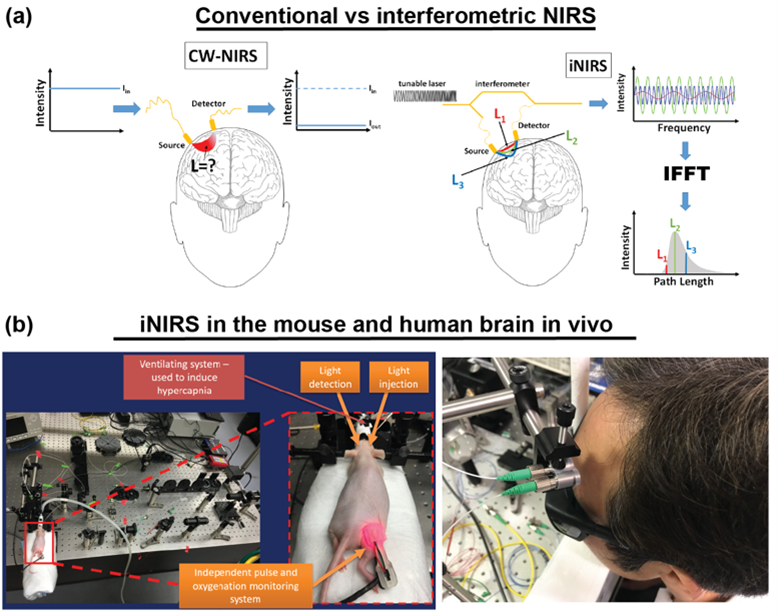
Image: Interferometry in brain monitoring.
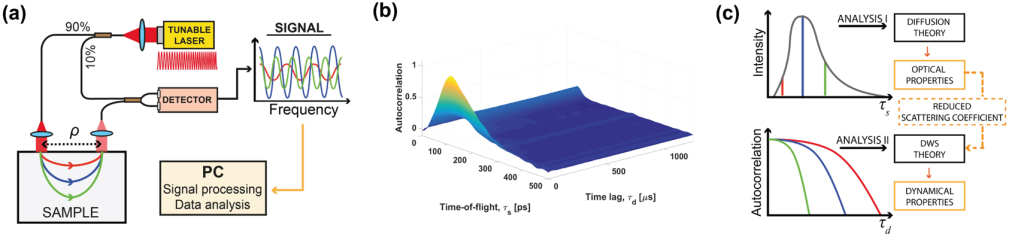
The optical phase is accessible in interferometric near-infrared spectroscopy (iNIRS). The iNIRS approach uses interferometry based on a temporally coherent tunable laser to achieve TOF resolution. Specifically, iNIRS supplements the conventional NIRS configuration with the tunable light source and the reference arm. The field remitted from the sample is recombined with the reference field. The beat frequency of the signal encodes photon path lengths (or times-of-flight). Short paths produce lower beat frequencies than long paths. Consequently, the photon time-of-flight distribution can be achieved by inverse-Fourier transforming the recorded signal. However, iNIRS provides much more information through the two-dimensional autocorrelation function (ACF) of the reemitted optical field. In iNIRS, the ACF is measured as a function of time lag with TOF resolution. This two-dimensional measurement (figure below) encodes information about the sample’s absorption, scattering, and blood flow index (BFI).
iNIRS was validated in in liquid phantoms [9], mouse brain [10], and human brain in vivo [11]. However, the original iNIRS (as DCS and TD-DCS) uses single-mode fibers for light collection, requiring integration times of 0.5-1 second. This time frame is too long, precluding the ability to detect rapid blood flow changes in the human brain that could be linked to neural signals.
To overcome those limitations, we recently proposed parallel interferometric near-infrared spectroscopy (πNIRS). In πNIRS we use multi-mode fibers for light collection and a high-speed, two-dimensional camera for light detection. Each camera pixel acts effectively as a single iNIRS channel. So, the processed signals from each pixel are spatially averaged to reduce the overall integration time. Moreover, interferometric detection provides us with the unique capability of accessing complex information (amplitude and phase) about the light remitted from the sample, which with more than 8000 parallel channels, enabled us to sense the cerebral blood flow with only a 10 msec integration time (∼100x faster than conventional iNIRS). We used such an approach to monitor the pulsatile blood flow in a human forearm in vivo. Also, we demonstrated that this approach could monitor the activation of the prefrontal cortex by recording the change in blood flow in the forehead of the subject while he was reading an unknown text [12].
Text: Dawid Borycki, PhD habil.
Team:
Dawid Borycki, PhD habil.
Michał Dąbrowski, PhD
Klaudia Nowacka, MEng
References:
- S. Samaei, P. Sawosz, M. Kacprzak, Z. Pastuszak, D. Borycki, and A. Liebert, “Time-domain diffuse correlation spectroscopy (TD-DCS) for noninvasive, depth-dependent blood flow quantification in human tissue in vivo,” Sci Rep 11, 1817 (2021).
- D. Borycki, O. Kholiqov, and V. J. Srinivasan, “Interferometric near-infrared spectroscopy directly quantifies optical field dynamics in turbid media,” Optica 3, 1471-1476 (2016).
- D. Borycki, O. Kholiqov, S. P. Chong, and V. J. Srinivasan, “Interferometric Near-Infrared Spectroscopy (iNIRS) for determination of optical and dynamical properties of turbid media,” Opt Express 24, 329-354 (2016).
- D. Borycki, O. Kholiqov, and V. J. Srinivasan, “Reflectance-mode interferometric near-infrared spectroscopy quantifies brain absorption, scattering, and blood flow index in vivo,” Opt Lett 42, 591-594 (2017).
- S. Samaei, K. Nowacka, A. Gerega, Z. Pastuszak, and D. Borycki, “Continuous-wave parallel interferometric near-infrared spectroscopy (CW piNIRS) with a fast two-dimensional camera,” Biomed Opt Express 13, 5753-5774 (2022).