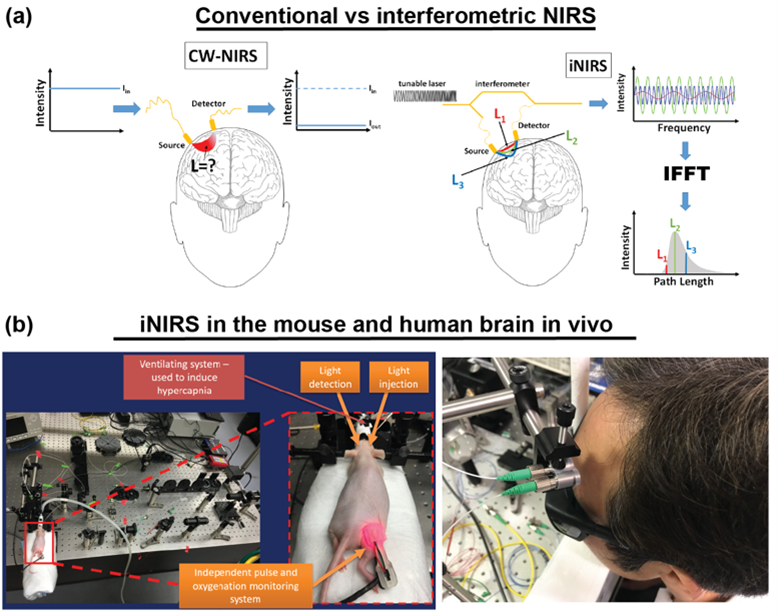
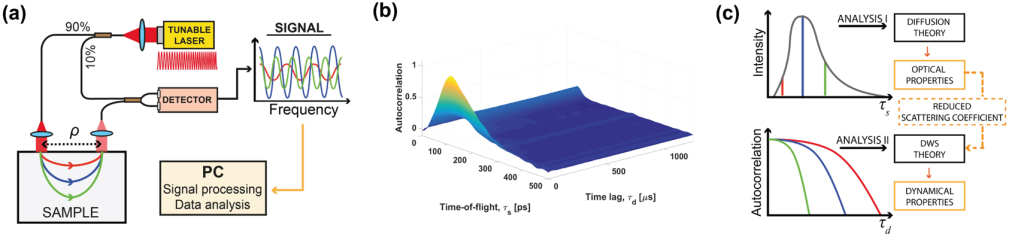
One of the primary initiatives within the IDoc Group focuses on the development of safer and more effective tools for eye surgery. This endeavor posed a distinct challenge for us, as it fell outside our traditional areas of expertise. Nevertheless, it is indeed a remarkable achievement that we have managed to assemble a team that, in under three years, has successfully amalgamated diverse expertise and advanced the project to its current stage. Our journey was marked by a gradual accumulation of knowledge and experience, ultimately culminating in the integration of all the components.
We are now pleased to inform you of the initial experiments where a robotic manipulator has been deployed to enhance manual ophthalmic surgical procedures. These innovations are complemented by the integration of Optical Coherence Tomography (OCT) images, meticulously aligned with the surgical tools’ tip precise position.
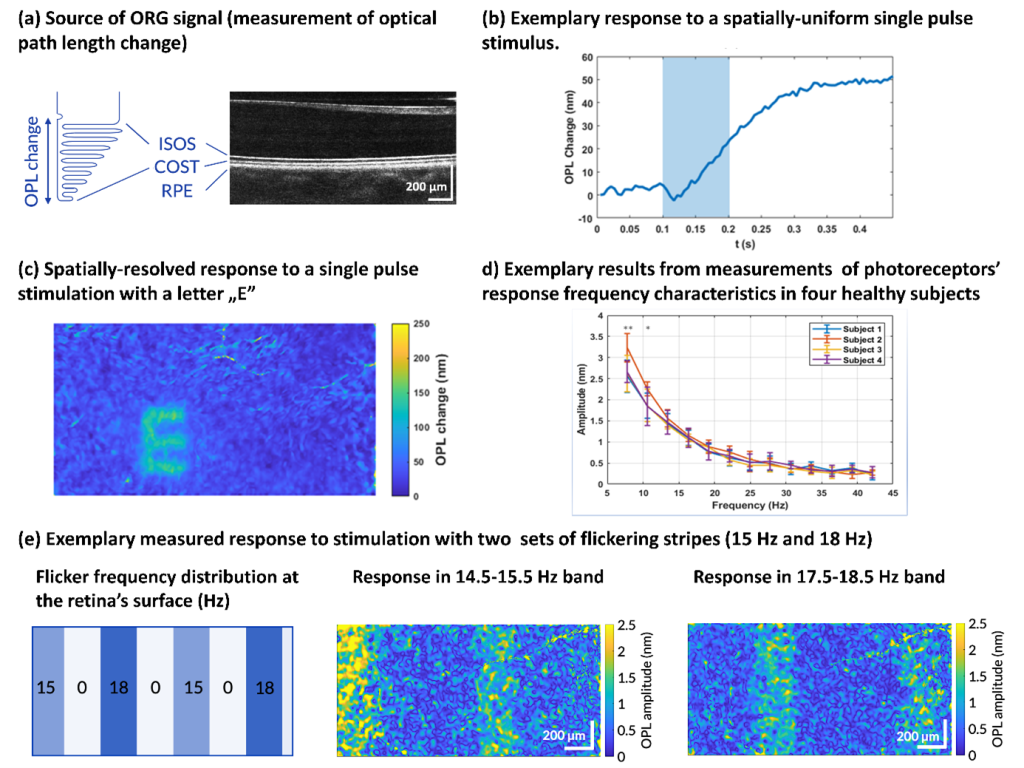
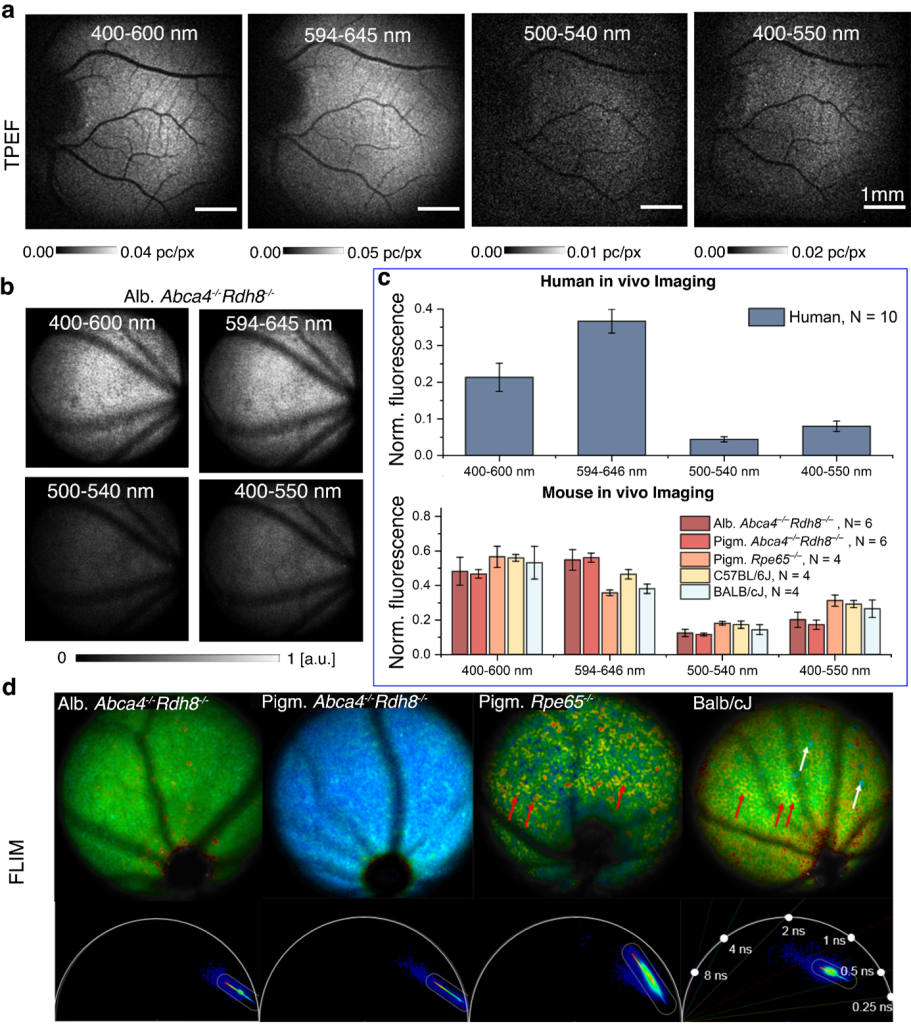
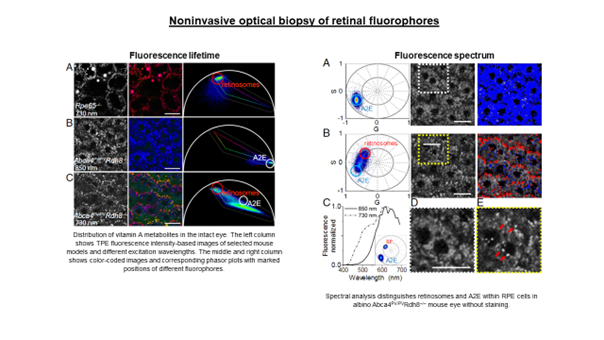
Another project the IDoc laboratory has been involved in goes to the very core of what the ICTER research centre aims to develop, that is methods and instrumentation to detect proper eye structure and function and their alteration in case of disease in an objective way. We did so in collaboration with the POB lab, by pioneering a technique called optoretinography. We are combining this with structural biology tools from the ISB lab for analysing the cellular machinery and its complex changes during the visual cycle in order to validate our hypotheses about what process the functional signal we measure originates from. To do so we are validating our functional imaging results with electrophysiology methods together with OBi laboratory.
It has been a challenging and ambitious project so far, but its collaborative nature made it all the more rewarding when not long ago we observed, in a repeatable way and for the first time, reduced functional responses from mice subject to temporal inhibition of vision, compared to their response only a couple of hours before the pharmacological treatment. We were able to objectively show with optoretinography that when a central protein (from the PDE family) involved in the phototransduction is inhibited, the mouse retinal photoreceptors, when exposed to a short flash of light, do not elongate nearly as much as they do when the mouse eye is fully functional. Measuring such a small physical change on photoreceptor length in vivo, as we are talking of only few tens of nanometers, can have a huge impact on vision science and ophthalmology by providing an objective functional test of visual ability and photoreceptor health. This in turns can speed up therapy selection and efficacy studies.
We look forward to upcoming results in this field.
Authors: